
The melting and boiling points for a given noble gas are close together, differing by less than 10 ☌ (18 ☏) that is, they are liquids over only a small temperature range. The properties of the noble gases can be well explained by modern theories of atomic structure: Their outer shell of valence electrons is considered to be "full", giving them little tendency to participate in chemical reactions, and it has been possible to prepare only a few hundred noble gas compounds. For example, argon is used in incandescent lamps to prevent the hot tungsten filament from oxidizing also, helium is used in breathing gas by deep-sea divers to prevent oxygen, nitrogen and carbon dioxide toxicity. The inertness of noble gases makes them very suitable in applications where reactions are not wanted. Noble gases are typically highly unreactive except when under particular extreme conditions. Because of the extremely short 0.7 ms half-life of its only known isotope, its chemistry has not yet been investigated.įor the first six periods of the periodic table, the noble gases are exactly the members of group 18. Although IUPAC has used the term "noble gas" interchangeably with "group 18" and thus included oganesson, it may not be significantly chemically noble and is predicted to break the trend and be reactive due to relativistic effects. Oganesson (Og) is a synthetically produced highly radioactive element. The six naturally occurring noble gases are helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). The elements placed in group 18 are called the noble gas electrons which have completely filled configurations and are highly stable and unreactive.The noble gases (historically also the inert gases sometimes referred to as aerogens ) make up a class of chemical elements with similar properties under standard conditions, they are all odorless, colorless, monatomic gases with very low chemical reactivity. Note: We should know the atomic number of the elements, so that we could write the electronic configuration of the atom and we should be thorough with the order of orbitals and number of electrons each shell can accommodate to write the configuration of atoms. Now let’s write the electronic configuration of carbon as, The carbon atom belongs to the second period of the periodic table. We know that the atomic number of carbon is 6.Therefore there will be 6 electrons present in its o-orbitals. Let’s solve the problem and get an idea and difference between the two types of representation of electrons in carbon. It is quite confusing to explain with the aid of words.
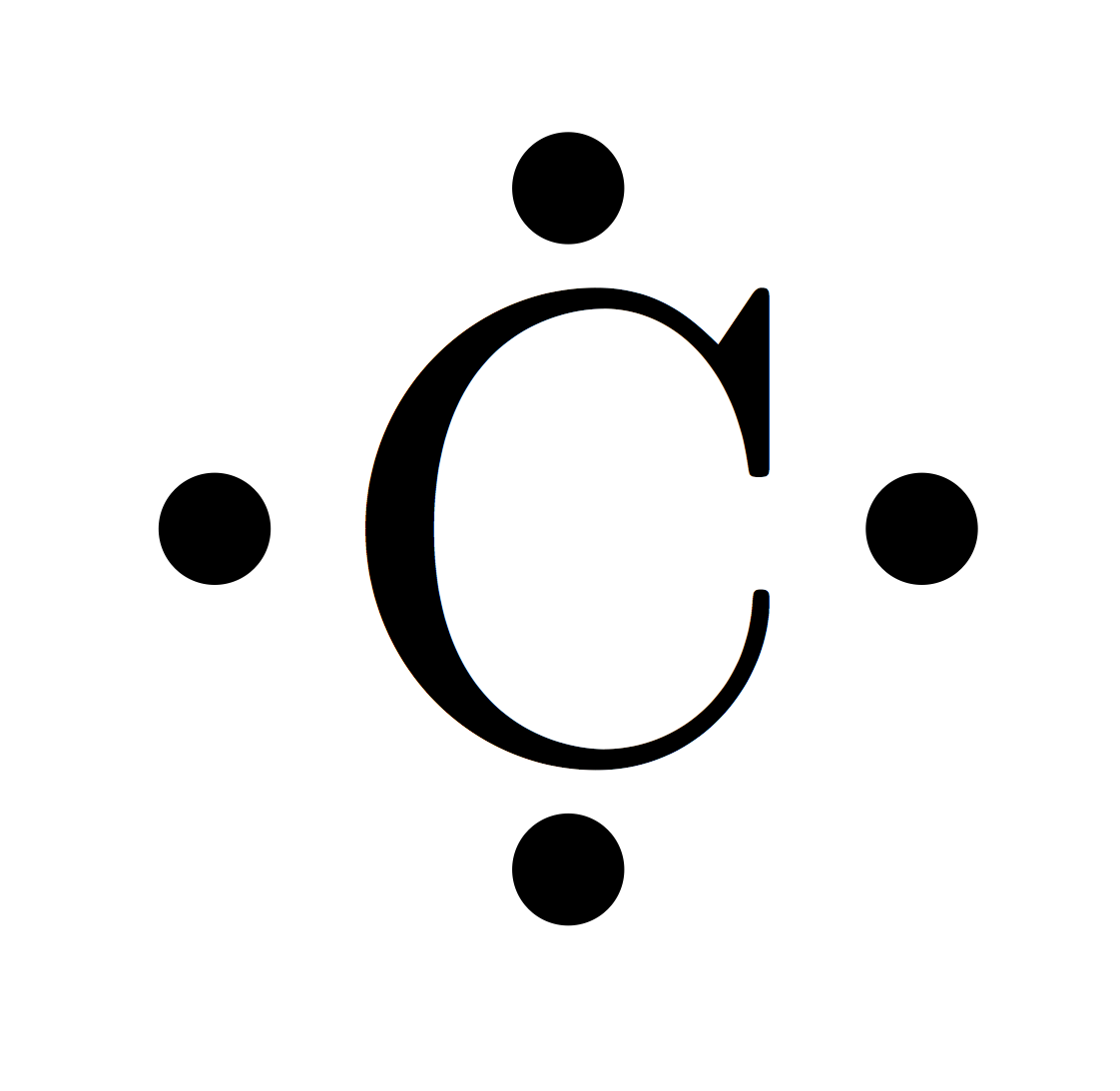
The electronic configuration is the representation of the total number of electrons in each orbitals of the atom in the increasing order of their energy.īut in noble gas electronic configuration, we will insert the chemical notation of the noble gas which is present in the previous period and write the further electrons present other than those present in the orbitals of the noble gas. To solve this problem we should know the difference between the electronic configuration and noble gas electronic configuration of an atom. Now in the question we are supposed to write the noble gas electronic configuration. And the atomic number of the element gives the number of electrons and protons in the atom whereas the atomic mass number is the sum total of the number of protons and neutrons in the atom. We know that the elements are placed in periodic take in accordance with the increasing order of the periodic table. From the lower classes we are very familiar with the elements and its atomic number and positioning in the periodic table. In the question it is asked how will be the noble gas configuration of a carbon atom. Trace the noble gas present in the previous period with respect to carbon.


 0 kommentar(er)
0 kommentar(er)
